1 Supercapacitor electrode materials-Conductive polymer
Supercapacitor electrode materials-Conductive polymers (conductive polymers, CP), also known as conductive polymers, are polymers with conjugated π bonds that are chemically or electrochemically doped or compounded to make them change from insulators to A class of polymer materials in the range of semiconductors or conductors. After doping, the band gap of conductive polymers will be narrowed, and electrons will more easily transition from HOMO (highest occupied molecular orbital) to LUMO (lowest unoccupied molecular orbital) , so as to improve its conductivity. There are two ways of doping, one is to use oxidizing or proton-donating substances to be incorporated into conductive polymers, and electrons are obtained from the HOMO energy level to form HOMO and LUMO The half-filled energy band between it and the energy difference between it and the LUMO energy level is reduced, which is called p-type doping; and another doping method is to use reductive material doping to provide electrons to the LUMO energy level, so that the LUMO The energy is reduced, thereby reducing the energy level difference between it and the HOMO, which is called n-type doping.
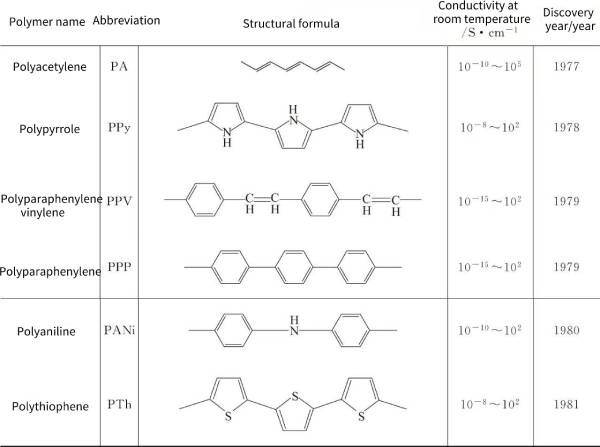
In 1977, Professor H.Shirakawa of Japan, Professor A.G.MacDiarmid and Professor A.J. Heeger of the United States discovered the metal-like conductive properties of polyacetylene (PA), which completely broke the view that organic polymers are insulators and broadened the scope of conductive polymers. and related research areas. Subsequently, other conductive polymers have been reported successively, such as polyaniline (PANi), polypyrrole (polypyrrole, PPy), polythiophene (polythiophene, PTh) and its derivatives, etc.
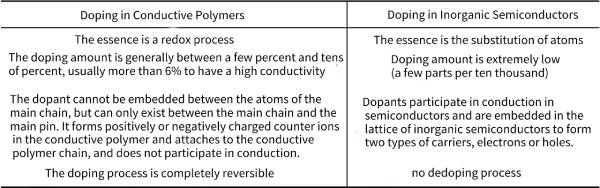
🌲The bandgap of intrinsically conductive polymers is usually 1.4~4.0eV, and the conductivity is usually in the range from insulator to semiconductor (10-10~10-4Scm-1), but it can be obtained after chemical or electrochemical doping. High, even metal-like electrical conductivity. Therefore, one of the biggest features of conductive polymers is that their electrical conductivity can be varied within a wide range of 10-10~10-5S·cm-1 by controlling doping. It is worth noting that “doping” in conductive polymers is very different from that in inorganic semiconductors. See Table 4-1 for specific differences.
The doped conductive polymer not only retains the diversification of polymer structure, processability and flexible mechanical properties, but also has the characteristics of semiconductor or conductor brought about by doping, which is a morphological change. The material with the largest span can realize the change from insulator to semiconductor and then to conductor. Compared with transition metal oxides or gas oxides, conducting polymers have attracted extensive attention due to their large theoretical specific capacitance, good electrical conductivity, low cost, and ease of large-scale production. In addition, conductive polymers have unique advantages as supercapacitor electrode materials:
1. The overall performance of the capacitor can be improved by designing the structure of the polymer and optimizing the matching of the polymer.
2. With the increase of the electrode potential, there is a continuous arrangement of oxidation states, which corresponds to the reversibility of the charge deintercalation and intercalation process.
3. Long service life, wide temperature range, fast charge/discharge and no charge/discharge control circuit.
4. There are a large number of microporous structures that can penetrate into the electrolyte inside and on the surface, and can form a network-like three-dimensional structure. The transfer of electrons and ions in the electrode material can be completed by exchanging with ions in the electrolyte.
5. Light weight, good elasticity, low cost, easy to prepare.
2 Energy storage mechanism of conductive polymer electrode materials
🌳The energy storage mechanism of conductive polymer supercapacitors is different from that of carbon materials. The energy storage of the latter is mainly achieved by electric double layer energy storage; while the former is mainly achieved by the fast Faradaic reaction of electrode materials under a specific voltage. To complete, also accompanied by the double layer effect, so it has a higher specific capacitance. That is to say, part of the capacitance of the conductive polymer supercapacitor comes from the electric double layer at the electrode/solution interface. During the charging/discharging process, the positive and negative ions in the electrolyte will be embedded in the polymer array to balance the charge of the polymer itself to achieve charge storage. The other part comes from the redox reaction during electrode charging/discharging. When the oxidation reaction occurs, the ions in the electrolyte enter the polymer skeleton; when the reduction reaction occurs, these ions that enter the polymer skeleton are released into the electrolyte. , thereby generating a current. This oxidation/reduction reaction not only occurs on the surface of the polymer, but also runs through the entire structure of the polymer. Since this charging/discharging process does not involve any structural changes in the polymer, the process is highly reversible. During the charging/discharging process, the conductive polymer with high electrochemical activity in the electrode undergoes reversible n-type or p-type doping or dedoping, so that it stores high-density charges and produces a certain scale of Faraday capacitance.
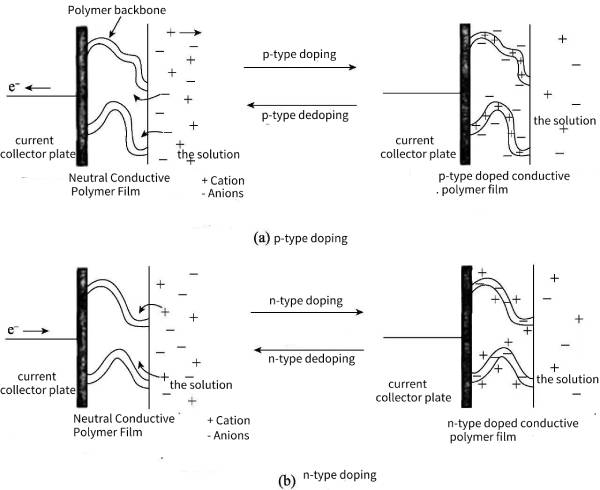
The p-type doping of conductive polymers means that the external circuit absorbs electrons from the polymer backbone, so that positive charges are distributed on the polymer molecular chains, and the anions in the solution will gather near the polymer backbone to maintain charge balance (such as poly aniline, polypyrrole and its derivatives), the specific process is shown in Figure 4-1 (a). The n-type doping of conductive polymers means that the external circuit transfers a large number of electrons and distributes them on the polymer molecular chain, making it rich in negative charges, so that the cations in the electrolyte gather near the polymer skeleton to maintain charge balance (such as polyacetylene, polythiophene and their derivatives), the specific process is shown in Figure 4-1 (b). However, there are few conductive polymers that can be effectively n-type doped, mainly because n-type doping is often unstable, and its own expansion and contraction functions may lead to its own degradation during cycling, thermal stability and cycle performance in long-term cycling Difference.
🌿Due to the different doping forms of conductive polymers and the types of conductive polymers that can be doped, different combinations of conductive polymers can be used as supercapacitor electrode materials. At present, there are three main types of supercapacitors based on conductive polymers:
(1) Type I (symmetric type) This type of supercapacitor is also called p-p type supercapacitor, and both electrodes use the same p-type doped conductive polymer. When the capacitor is fully charged, the polymer on the cathode is in an undoped state, and the polymer on the anode is in a fully doped state; during the discharge process, the cathode in the undoped state undergoes an oxidation doping reaction, while the polymer in the doped state The anode polymer is reduced (dedoped). When it is discharged until both electrodes are in a semi-doped state, the voltage difference between the two electrodes is zero. It can be seen that the amount of charges released during the discharge of type I capacitors is only 1/2 of the fully doped charges, and the potential difference between the two poles is small (about 1V). Therefore, the operating voltage of type I supercapacitors is low, generally lower than 1.0V, which limits its energy density. Although type I capacitors have some drawbacks, research on this type of supercapacitor is still ongoing due to the fact that most conducting polymers can be p-type doped and the electrode assembly is relatively simple.
☘️(2) Type II (asymmetric type) This type of supercapacitor is also called p-p’ type supercapacitor. The two electrodes are composed of different types of conductive polymers that can be p-type doped. The potential range over which the two conductive polymer electrodes are doped is different, allowing the capacitor to have a higher voltage difference (typically 1.5V) in a fully charged state. During the discharge process, the dedoping rate of the anode p-type doped conductive polymer is greater than 50%, which makes the electrode have a larger discharge capacity. Compared with type I capacitors, the operating voltage of type II capacitors can be increased to 1.5V, and the energy density is improved.
(3) Type III (symmetric type) This type of supercapacitor is also called n-p type supercapacitor. The two electrode materials of the capacitor are composed of the same conductive polymer that can be doped in both n-type and p-type. In the charging state, the cathode of the capacitor is in a fully n-type doped state, while the anode is in a completely p-type doped state, so that the voltage difference between the two electrodes is further increased (3~3.2V), and the doped charge can be released during the discharge process. All released, greatly improving the capacitance of the capacitor. This type of supercapacitor has a high charge utilization rate, and the potential window can be as high as 3.0V. When charging, both electrodes are doped, the charge storage capacity is large, and the conductivity is high. Therefore, type III supercapacitors are currently the most promising one. an energy storage device.
3 Types of conductive polymer electrode materials
🍀There are many types of conductive polymers, and conductive polymers can usually be divided into two categories: one is composite conductive polymers; the other is structural conductive polymers.
3.1 Composite conductive polymer
Composite conductive polymer refers to the polymer structure material with poor conductivity as the matrix (continuous phase), and various conductive fillers (such as carbon-based materials, metals, metal oxides, etc.) Composite materials with certain electrical conductivity and good mechanical properties are obtained by compounding, surface compounding or gradient compounding. This type of composite conductive polymer, its intrinsic polymer has good mechanical properties, can be well combined with conductive fillers, endowing the material with better stability, and its electrical conductivity is mainly provided by the conductive fillers. flow to complete.
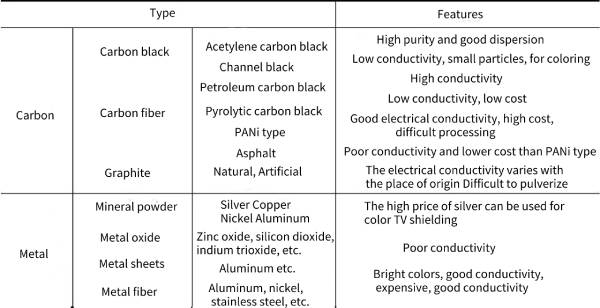
🎍There are mainly two types of composite conductive polymers: one is filled composite conductive polymer, which is to compound the polymer matrix with various conductive fillers; the other is blended composite conductive polymer, which is to combine The polymer matrix is blended with a structurally conductive polymer. Table 4-2 summarizes the classification and characteristics of conductive substances commonly used in composite conductive polymers.
3.2 Structural conductive polymers
Structural conductive polymers, also known as intrinsically conductive polymers, refer to a class of polymers that can provide carriers themselves, or have conductive functions after being “doped”. .This type of conductive polymer is generally a conjugated polymer. In a conjugated polymer, the valence electrons do not contribute to the conductance. On the other hand, due to the influence of the chain regularity, the degree of polymerization n is often small, so that It is difficult for electrons to transition from p orbital to p’ at room temperature, so the conductivity is low. According to the energy band theory, if the energy band region is partially filled, conductance can be generated, so reducing electrons in the valence band (p-type doping) or injecting electrons into the empty energy band region (n-type doping) can achieve partial filling of the energy band , resulting in a conductance phenomenon.
🎋According to different conduction mechanisms, structural conducting polymers can be divided into three categories: ion conducting polymers, electronic conducting polymers and redox conducting polymers.
3.2.1 Ionically Conductive Polymers
Ionically conductive polymers are conductive polymers in which anions and cations are the main carriers. Above the glass transition temperature, the physical properties of the polymer change significantly. It is similar to a high-viscosity liquid and has certain fluidity. A certain degree of directional diffusion movement occurs in the polymer, which makes the polymer conductive and exhibits the properties of an electrolyte. As the temperature increases, the fluidity of the polymer becomes more prominent, and the conductivity is also improved. Our so-called polymer solid electrolytes and polymer ion conductors are ionically conductive polymers. Ionic conductive polymers mainly include complexes formed by polyester and metal salts and complexes formed by polyaldehydes and alkali metals, etc.
3.2.2 Electronic Conductive Polymers
🍃Electronic conductive polymers are also called conjugated conductive polymers, and their carriers are free electrons or holes. Electronically conductive polymers are characterized by a large linear conjugated π structure in the molecule, which provides a prerequisite for the delocalized migration of carriers. The π valence electrons have a large delocalization property and can relatively migrate within the system. When there is an external electric field, the T valence electrons inside the material flow directionally to generate current, showing the phenomenon of electronic conductors. There are many types of electronic conductive polymers, such as aliphatic linear conjugated polymers such as polyacetylene (PA) and polyoxyethylene (PEO), and aromatic linear conjugated polymers such as polyaniline (PANi) and polycarbazole (PCA). type conjugated polymers and aromatic heterocyclic linear conjugated polymers such as polypyrrole (PPy) and polythiophene (PTh). This kind of material has attracted many researchers to develop it because of its excellent performance.
3.2.3 Redox conductive polymers
Redox-type conductive polymer, which uses redox reaction as the mechanism of electron transfer. In the reversible redox reaction, electrons can move directionally between molecules so that the conductive polymer has the ability to conduct electricity. The prerequisite for this type of conductive polymer is that the structure needs to contain an active body that can undergo reversible redox reactions, such as polyethylene ferrocene, etc.
🍂Table 4-3 summarizes the structure and conductivity of typical structural conducting polymers.
(1) Polyacetylene (polyacetylene) is referred to as PA. In 1967, Japanese chemist Hideki Shirakawa accidentally synthesized silver-white polyacetylene with metallic luster in the laboratory. It is the first polymer discovered to conduct electricity, with repeating (C2H2)n structural units. The discovery of PA has greatly promoted the rapid development of conductive polymer research
🍁(2) Polypyrrole (polypyrrole), PPy for short, is an important electronically conductive polymer, and is a heterocyclic conjugated conductive polymer that is currently researched and used more. It is usually an amorphous black solid, and its structural formula is shown in Figure 4-2:
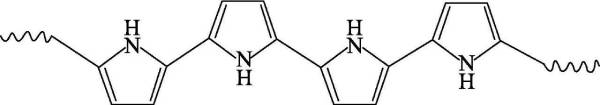
The heteroaryl and extended p-conjugated backbone structures of PPy provide chemical stability and conductivity, respectively. However, the p-conjugated backbone structure is not sufficient for appreciable conductivity on its own, and partial charge extraction from the PPy chains is required, which is achieved through a chemical or electrochemical process called doping. The conductivity of neutral PPy is dramatically changed from the insulating state to the metallic state by doping, a very valuable feature for applications where the conductivity of the material must be controlled. Studies by Frackowiak et al. have shown that the capacitance of a capacitor made of a carbon nanotube-conducting polymer (such as polypyrrole) composite material as an electrode material for a supercapacitor is higher than that of pure carbon nanotubes or pure polypyrrole. Wang et al. prepared nanofiber-bonded PPy/graphene oxide paper by in-situ polymerization for supercapacitors, which still showed a large specific capacity of 198F·cm-3 after 16,000 cycles at 5A·g-1.
🍄(3) Polythiophene (polythiophene) referred to as PTh, the polymer has excellent environmental and thermal stability in its neutral and doped state, and exhibits excellent optical properties and electrical conductivity as high as 600S·cm-1 in the doped form value. Polythiophene has poor solubility in most organic solvents, and its use is limited except for mixtures such as arsenic trifluoride/sulfur pentafluoride. However, the addition of long flexible alkyl side chains at the 3-position of the thiophene ring has been reported to produce soluble polymers in conventional organic solvents without changing the chemical and physical properties of the polymers.
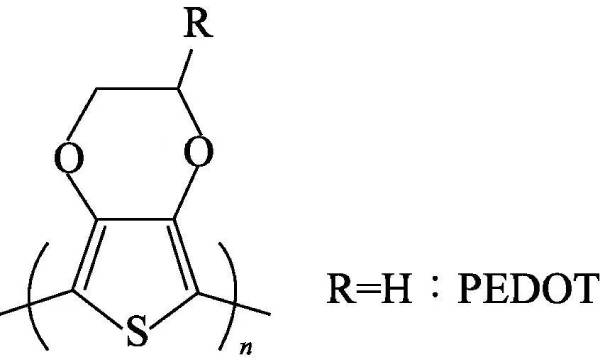
In 1980, unsubstituted polythiophene was prepared for the first time by Japan’s Yamamoto and its collaborators using metal nickel compounds as catalysts. Metal Mg and 2,5-dibromothiophene were formed in tetrahydrofuran solution by catalyst Ni (bipy) CI2. Dimer thiophene is finally polycondensed to obtain macromolecular polythiophene, and its structural formula is shown in Figure 4-3. Then they also synthesized polythiophene with methyl, hexyl, octyl and dodecyl respectively substituted at the 3-position by nickel compound catalysis.
🐚(4) Polyaniline (polyaniline) referred to as PANi, since it was redeveloped by Macdiarmid in 1984, with its good thermal stability, chemical stability and electrochemical reversibility, excellent electromagnetic microwave absorption performance, potential solution and melting Processability, easy access to raw materials, simple synthesis methods, and unique doping properties have made it one of the fastest-growing conductive polymer materials. The chemical structural formula of polyaniline is shown in Figure 4-4:
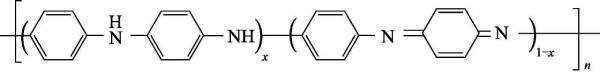
Polyaniline can be regarded as a copolymer of phenylenediamine and quinonediimine, and x represents the content of a certain state of polyaniline. When x=0, it is a fully reduced polyaniline (pernigraniline, PNA); when x When =1, it is the polyaniline in the fully oxidized state (leucoemeraldine, LM); when x=0.5, it is the polyaniline in the intrinsic state (emeraldine, EM); when x=0.75, it is in the oxidation state: reduced state=3 :1 polyaniline (nigraniline, NA).
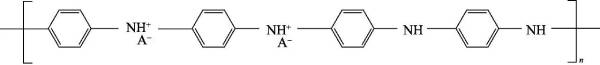
🌹Polyaniline has good electrochemical reversibility and can be freely converted between the above three forms, but it is non-conductive in its intrinsic state, and it is only conductive after being doped with a protonic acid. The doping process is obviously different from that of polyaniline. other conductive polymers. When doping, there is no change in the number of electrons on the molecular chain of polyaniline (as shown in Figure 4-5), but protonation occurs on the imine nitrogen atom, generating polarons and making the doped band of the molecular chain Holes appear on the surface, that is, p-type doping occurs.
Depending on its oxidation state, PANi exists in the following species: white anthocyanins, emeraldines and o-phenylenediamines, but only protonated emeraldines are conductive, while doped white anthocyanins and o-phenylenediamines Amines have poor conductivity, for the remeth . synthesized polyaniline nanospheres using spherical structure metal oxide Mn3O4 as an active seed template (as both a template and an oxide). Fe et al. used porous layered nanostructured MnO2 as an active seed template to synthesize alde-shell re-aniline spherical and structures. Wan Meixiang et al. prepared soluble polyaniline by emulsion-extraction method. The aniline monomer was polymerized in a liquid system formed by water and surfactant DBSA, and then the soluble PANi-DBSA was directly extracted with chloroform. This method has simple synthesis steps and high conductivity of the product. In addition, the group B also used -forbidden sulfonic acid (B-NSA) as a doping acid and template agent to prepare micro/nanostructured polyaniline by self-assembly method. Table 4-4 summarizes the advantages and disadvantages of PANi, PPy, and PTh-based conductive polymers.
4 Synthesis of conductive polymer electrode materials
🌺As an electrode material for supercapacitors, conductive polymers can be synthesized by many methods, the most commonly used methods are chemical synthesis and electrochemical synthesis. Chemical methods are simple, low in cost, and easy to carry out large-scale production, mainly including template method, emulsion polymerization method, interfacial polymerization method, dilute solution polymerization method, rapid mixing polymerization method, etc.; electrochemical methods are effective by selecting appropriate electrochemical parameters. Controlling the size and shape of the polymer, this method is suitable for the synthesis of conductive polymers in small batches due to the limited electrode area.
4.1 Chemical synthesis method
🌸Chemical oxidative polymerization generally uses oxidants in acidic media to oxidatively polymerize monomers. Commonly used oxidants include persulfate, hydrogen peroxide, ferric chloride, potassium dichromate, etc.; media often use sulfuric acid, hydrochloric acid, and perchloric acid. liquid. The type and concentration of the medium acid, the type, concentration, dosage, addition speed and reaction temperature of the oxidant have a direct impact on the properties of the final conjugated conductive polymer. Chemical oxidation polymerization can be used to directly prepare conductive polyaniline, conductive polypyrrole, conductive polythiophene, etc., and the obtained products are mostly polymer powders. Mallouki et al. deposited PPy on Fe2O3 by in-situ chemical polymerization to obtain composite materials. The specific capacities in EMITFSI and PYR14TFSI ionic liquids were 210F·g-1 and 190F·g-1, respectively, and the specific capacity faded only after 1000 cycles. 3%~5%. Liu Zhen et al. polymerized spherical PPy particles on the surface of GNS by oxidative in-situ polymerization method, and dispersed them uniformly on the surface of GNS to prepare PPy/GNS composite materials. The uniform dispersion of PPy on the surface of GNSs improves the electrical conductivity of the composite, which facilitates the diffusion of electrolyte ions and enhances the performance of the material. At a current density of 0.5A·g-1, the capacitive performance of the composite material can reach up to 402F·g-1, and its specific capacity decreases by about 5% after 1000 charge/discharge cycles, and both specific capacity and cycle stability are obtained. improve.
4.2 Electrochemical synthesis method
🌼The electrochemical method is to polymerize monomers on the electrode surface through electrochemical oxidation or reduction reactions under the action of potential. In this method, the electrode potential is used as the initiation and reaction driving force of the polymerization reaction, and the polymerization reaction is carried out on the surface of the electrode to directly generate a conductive polymer. Electrochemical preparation of conductive polymers has many advantages, mainly in the easy control of reaction conditions, high product purity, good mechanical properties and electrical conductivity, etc. Since the polymerization process does not require the introduction of oxidants, the electrochemical polymerization method is clean and environmentally friendly. At the same time, the electrochemical preparation method can also make the polymerization and doping proceed simultaneously. Many heterocyclic conductive polymers, such as polypyrrole and polythiophene, can be prepared by electrochemical methods. Roberts et al. prepared bithiophene-triarylamino conductive polymer on the surface of gold electrode by electrochemical deposition. The research results showed that the specific capacitance of the polymer in organic electrolyte was 50mV·s-1. The research result of up to 990F·g-1 is much higher than the specific capacitance of the usual activated carbon-based materials. Huang et al. prepared PANi/SWNTs composites by electrochemical polymerization. They proposed that SWNTS play a certain role in the polymerization process. The effect can promote the delocalization of charges between PANi and SWNTS, and promote the charge transfer, thereby improving the electroactivity and electrochemical performance of the composite material.
4.3 Photochemical method
The photochemical method is simple in operation, fast in response, low in cost and non-destructive to the surrounding environment. This method is useful for making some conductive polymers. For example, pyrrole is efficiently polymerized into polypyrrole by irradiation with visible light using a photosensitizer or a suitable electron acceptor. Currently, the polymerisation reaction of aniline via oxidative radical coupling in the presence of peroxide gas is initiated by horseradish peroxide. Polymerization of aniline by photochemical methods can be carried out under ambient mild conditions compared with chemical and electrochemical techniques.
4.4 Metathesis
🌻Metathesis is a chemical reaction between two compounds whereby two different compounds are formed by partial interchange of components of the two reactants. Metathesis polymerizations fall into three categories: ring-opening metathesis of cyclic alkenes, metathesis of alkynes, and metathesis of acyclic or cyclic dienes.
4.5 Concentrated emulsion method
The emulsion polymerization method is one of the most important methods for synthesizing high polymers. According to the reaction mechanism of free radical polymerization, the polymerization process can be divided into four stages—dispersion stage, latex particle generation stage, latex particle growth stage and polymerization reaction. complete stage. In the dispersion stage, the emulsifier molecules exist in three forms—dissolved in the aqueous phase as a single-molecule emulsifier, form micelles, or be adsorbed on the surface of monomer droplets. The monomer added to the system also has three destinations, that is, existing in the monomer droplet, dissolving in the aqueous phase in the form of a single molecule, and being compatibilized in the micelles. Typical emulsion polymerization is precisely defined as a polymerization method in which the polymer is preferentially formed within micelles.
4.6 Plasma polymerization
🌞Plasma polymerization is a new method for fabricating thin films from organic and organometallic preparations. Plasma-polymerized films are pinhole-free and highly cross-linked, thus insoluble, thermally stable, chemically inert and mechanically strong. In addition, the films are remarkably coherent and adhere to a range of substrates consisting of conventional polymer, glass and metal surfaces. Due to these excellent properties, they have been widely used in a series of applications such as Alfa selective membranes, protective shells, biomedical materials, electronics, optical devices, and adhesive supports over the past few years.
5 Application of Conductive Polymers in Supercapacitors
Compared with expensive metal oxides, conductive polymers have reversible faradaic redox properties, high charge density and low cost, and are widely used as electrode materials. At present, various forms of conductive polymers have been successfully synthesized And applied in supercapacitors, these nanostructured conductive polymers with high surface area and high porosity have good performance, especially one-dimensional nanostructured conductive polymers have very high pseudocapacitive properties
🌝The ultimate goal of researching conductive polymers is their incorporation into supercapacitor devices. With the emergence of new electrical devices such as portable devices and scrolling screens, there is an increasing demand for flexible, lightweight and advanced energy storage devices. Conductive polymers are considered to be one of the most promising electrode materials for flexible supercapacitor applications due to their high flexibility and ease of fabrication. In order to improve the electrochemical performance of supercapacitors based on It is very important to improve its crystallinity, control its microstructure and surface morphology by controlling the content of impurity agents, the type and content of surfactants, etc. In addition to electrochemical properties, other important properties of conductive polymers, including thermal stability, processability, and mechanical properties, should also be considered to meet the needs of practical applications.
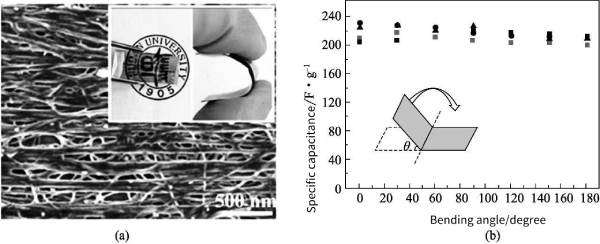
Polyaniline (PANi) has various structures, unique doping mechanism, good air stability, electrical conductivity, electrochromic properties, etc., so it has been a research hotspot as a conductive polymer with excellent performance. However, ions in PANi Diffusion within the molecular chain will lead to changes in its mechanical properties; at the same time, when PANi is used as an electrode material, the rapidly decaying specific capacitance limits its further application and development. One of the ways to solve this problem is to polymerize PANi on the surface of various carbon-based materials to obtain PANV carbon composites. The high specific surface area of carbon-based materials provides a reaction site for the deposition of PANi and increases the electroactive area. Zhang et al. successfully deposited PANi onto vertically aligned CNTs for the preparation of supercapacitors, and obtained a high-quality normalized specific capacitance of 1030F g-1. In addition to oxidative chemical polymerization methods, CNT/polymer nanocomposites can be efficiently fabricated onto conductive flexible substrates by electropolymerization methods. Recently, Lin et al. synthesized PANi/MWCNT composite films with good elasticity by electrochemical methods. The flexible composite electrode exhibits high specific capacitance and exhibits high stability up to 180° bending angle (Fig. 4-6).
☀️As one of the four most common conductive polymers, polypyrrole (PPy) has the advantages of good air stability, easy electrochemical polymerization to form films, and non-toxicity. It has broad application prospects, so it has been valued for more than 20 years. However, in the process of doping/dedoping, the polypyrrole molecular chain is prone to expand or shrink, resulting in the molecular chain structure being easily destroyed, which greatly reduces the actual value of the material. Oliveira et al. used methyl orange as a template to polymerize PPy nanotubes on its surface, and then used hollow PPy nanotubes as a matrix to cover the surface with a cross-linked network of single-walled carbon nanotubes (SWNTS) to form a PPY/SWNTS core-shell structure system. , and mixed with TiO2 at the same time, the nanotube-shaped PPY/SWNTS/TiO2 composite material was prepared, which strengthened the electric double layer effect and Faraday reaction of the material during the charge/discharge process, and the specific capacitance of the composite material was measured after the electrochemical performance test The highest value is 281.9F·g-1. Yanik et al. successfully prepared nanocomposites based on polypyrrole/graphene and magnetic polypyrrole/graphene, and the obtained nanocomposites were used to prepare conductive inks to manufacture supercapacitor batteries. According to CV analysis, the magnetic polypyrrole/graphene battery has a high specific capacitance value. With the influence of magnetic nanoparticles, the specific capacitance increases by about 12%, and its maximum specific capacitance can reach 255F·g-1
🌈Polythiophene (PTh) is used in supercapacitor electrode materials mainly by modifying thiophene to prepare corresponding electrode materials. Wang Hongmin and others mixed multi-walled carbon nanotubes (MWNTS) and PTh in different mass ratios to make polyaniline/multi-walled carbon nanotube composites, and tested that the electrical conductivity increased with the increase of MWNTS content after compression under a pressure of 14 MPa. When the content of MWNTs was 3%, the electrical conductivity of the composite reached 6.61×10-6S cm-1, and when the content of MWNTs increased to 20%, the increase rate of electrical conductivity was relatively slow, and gradually approached that of pure MWNTs and reached a constant value. Han Feifei et al. ultrasonically blended MWNTs and P3OT (poly-3-octylthiophene) powder in chloroform solution for 15 minutes according to different mass ratios, dried at a constant temperature of 50°C, mechanically ground, and tested its conductivity after constant pressure tableting. The electrical conductivity of the composites when the content of pure P3OT and MWNTs is 3% is 4.14×10-15s·cm-1 and 1.43×10-2s·cm-1, respectively. The reason for the increase in the conductivity of the composite material is that MWNTs is a conjugated polyene structure, and the π electrons have a strong delocalization, which can produce π-π conjugation with the π electrons on the main chain of the thiophene ring to form a larger The conjugated system makes the electrons have a larger delocalization space.




