Electrolyte of aluminum electrolytic capacitor—the working electrolyte of capacitor is another key factor in addition to corrosion and empowerment. It is related to the operating temperature range of aluminum electrolytic capacitors, as well as working indicators such as life and reliability. There are generally two types of working electrolytes: liquid electrolytes and solid electrolytes.
1 Electrolyte of aluminum electrolytic capacitor – liquid electrolyte
1.1 Electrolyte of Aluminum Electrolytic Capacitor—Property requirements of working electrolyte
Since the energizing liquid only contacts the device during the energizing process, while the working electrolyte is always in contact with other parts after the capacitor is manufactured, there is no chance to replace it again, and it works at any time during the working process, and its involvement is wider. Therefore, There are stricter requirements on the properties of the working electrolyte than the energizing solution. details as follows:
(1) When the working electrolyte comes into contact with the base metal of the anode and cathode foils and their oxide films, electrolytic paper, inner wall of the casing, sealing materials, etc., no reaction, corrosion, outgassing, etc. can occur.
(2) The working electrolyte must have high oxidation efficiency and can continuously provide electrochemical ability to repair weak spots and damaged areas of the dielectric oxide film during operation.
(3) The resistivity and viscosity of the working electrolyte should be as small as possible, without drastic changes within the specified temperature range, and in good contact with the dielectric film.
(4) The working electrolyte should not freeze at low temperatures and not easily vaporize at high temperatures.
(5) The working electrolyte must have good permeability to the corrosion holes and cracks of the aluminum foil and the electrolytic paper.
(6) The working electrolyte needs to have low vapor pressure and will not dry up due to evaporation or leakage from the shell seal.
(7) The flash voltage of the anode foil in the working electrolyte should be higher than the working voltage of the capacitor.
(8) The working electrolyte must have extremely high purity, and the content of impurity ions such as C1m– and SO 42-must be strictly limited so as not to gradually destroy the dielectric film and electrode foil.
(9) The working electrolyte must have high stability and must not be flammable, toxic or other characteristics that may harm the operator.
(10) The purchase price of working electrolyte should not be too high.
It is quite difficult to obtain a working electrolyte that meets the above requirements at the same time. Therefore, when selecting an electrolyte, it is generally necessary to give priority to ensuring that some of the requirements are met based on the type of electrolytic paper, the structure and working conditions of the capacitor. For example, the electrolyte used in low-voltage capacitors should give priority to low viscosity and low resistivity, while the electrolyte used in high-voltage capacitors should have the primary goal of not reducing the flash voltage, and then try to reduce the resistivity and viscosity as much as possible while meeting the flash voltage.
Electrolyte of aluminum electrolytic capacitor—the above is an analysis of the property requirements of the working electrolyte
1.2 Electrolyte of aluminum electrolytic capacitor -Composition of working electrolyte
The working electrolyte is mainly composed of solute (electrolyte) and solvent, but sometimes it is necessary to add some additives according to certain requirements, such as viscosity, resistivity and extended temperature range, preventing oxide film corrosion or cathodic polarization, etc.
1. Electrolyte of Aluminum Electrolytic Capacitor—Requirements for solute and solvent
(1) The solute in the electrolyte is used to maintain the operation of the vaporization film. When the damage to the oxide film is not too serious, the solute will be repaired during the working process. Therefore, it must have the electrical conductivity required by the voltage, be easily soluble in solvents, and have strong e-commerce capabilities to ensure that it has sufficient repair capabilities. In addition, it is also necessary to consider that the solute is corrosive to the base metal and the generated oxide film, such as sulfuric acid, oxalic acid, etc. Although its ionization ability is very strong, it cannot be used as a solute.
Therefore, generally weaker inorganic or organic acids are used as solutes. On the other hand, due to the low degree of dissociation of these acids and the low concentration pH value, an appropriate amount of alkali, such as ammonia and organic amines, must be added to neutralize it with the acid to form salts, which cannot adjust the pH value. , and can also improve the conductivity.
(2) The solvent of the electrolyte is the basis of the electrolyte and the key to determining the working temperature range of the product. Therefore, its boiling point and freezing point are very important. Its boiling point should be higher than the upper limit of the working temperature, and its freezing point should be lower than the lower limit of the working temperature. , ensuring that the solvent remains liquid throughout the entire operating temperature range. Secondly, the change in viscosity of the solute with temperature should also be small, and the temperature characteristics of the resistivity should be excellent. In addition, requirements such as low vapor pressure, low viscosity, good solubility, higher polarity and smaller dielectric loss must also be considered.
2.Acid used in working electrolyte
The most commonly used acids in working electrolytes are inorganic weak acids (such as boric acid, phosphoric acid), or various carboxylic acid compounds with carboxyl (-COOH) functional groups, such as formic acid, lactic acid, benzoic acid, malonic acid, and maleic acid. acid, adipic acid, sebacic acid, dodecanedioic acid, hydrogenated titanic acid, citric acid, etc. In addition to carboxylic acids, organic acids with phenol properties, such as nitrophenol, or organic acids with an acid group (-SO3:H), such as sulfamic acid, are sometimes used.
Acids are generally responsible for providing anionic groups in the electrolyte.
3. Alkali used in working electrolyte
Because the electrolyte is required to be weakly acidic and has a weak corrosive effect on the oxide film, it is generally necessary to add alkali to the acid to adjust the pH value and resistivity. At the same time, adding alkali can also improve the solubility of the solvent, which is beneficial to the preparation of the electrolyte.
Most of the time, the added alkali is ammonia water, which reacts with acid to form ammonium salts. The ammonium salts are less corrosive to A12O3 than other inorganic alkali salts. In addition to inorganic bases, alkaline organic compounds, such as various organic amines, are sometimes added. When selecting a base, it must be ensured that the salt generated by neutralization with the acid can be dissolved in the selected polar solvent and produce the required anionic and cationic groups.
The base is generally responsible for providing cationic groups in the electrolyte.
4. Solvent used in working electrolyte
Generally, the stronger the polarity of the solvent, the stronger the dissolution and ionization of the electrolyte, but the greater the viscosity, and the easier it is to solidify at low temperatures; conversely, the weaker the polarity of the solvent, the worse the dissolution and ionization of the electrolyte, but the viscosity The smaller it is, the lower the vapor pressure at high temperatures. Therefore, the solvent of the working electrolyte is usually a mixture of two or more solvents, which can learn from each other’s strengths, adjust the viscosity, improve stability, and improve high and low temperature characteristics. For example, mixing water and ethylene glycol in a mass ratio of 35:65 can greatly improve the low-temperature characteristics, and the freezing point can be reduced to -70°C.
Commonly used solvents include alcohols, ethers and amides.
1)Alcohols
Monohydric alcohols, such as propanol, diacetone alcohol, and benzyl alcohol.
Glycols, such as ethylene glycol, diethylene glycol, hexanediol, and phenylhexanediol.
Trihydric alcohols, such as glycerol, 3-methyl 1,3,5-pentanetriol.
Six-valent alcohols, such as mannitol and sorbitol.
2) Ethers
Monoether, such as diethyl ether.
Ethylene glycol monoalkyl ether, such as ethylene glycol monomethyl ether, ethylene glycol monoethyl ether.
Ethers of diethylene glycol, such as diethylene glycol monomethyl ether, diethylene glycol monoethyl ether, and diethylene glycol dimethyl ether.
Other ethers, such as ethylene glycol monophenyl ether.
3) Amides
Primary amide with RCONH2 structure, replacing one hydrogen atom, such as formamide, N-methylformamide, N,N-dimethylformamide, N,N-diethylformamide, N,N-dimethyl acetamide.
4)Other organic solvents
Other organic solvents such as dimethyl sulfoxide, gamma-butyrolactone, propylene carbonate, dioxyethane and methoxypropane.
5. Water in working electrolyte
Water is a high-quality solvent with strong ionization ability, which can reduce the resistivity of the electrolyte. In addition, water has good wettability to the electrode foil and electrolytic paper, and the price is low. Therefore, many electrolytes use solvents mixed with water. However, the following problems will occur when adding water, so it is not suitable for specific working environments:
(1) The aqueous electrolyte has a low boiling point and a large vapor pressure at high temperatures; at high temperatures, water will interact with aluminum and aluminum oxide films on both the positive and negative electrodes, which will increase the thickness of the surface oxide film and reduce the capacitance; water The insulation performance of Al0, deteriorates, the leakage current of the capacitor greatly increases, and the tangent value of the loss angle becomes larger; under high temperature, water continuously generates steam and accumulates in the capacitor, causing the internal pressure to rise, and there is a risk of bursting. Therefore, aqueous electrolytes are not suitable for high temperature environments.
(2) Electrolytes containing more water have lower flash voltages and cannot be used as high-voltage electrolytes. Due to the high conductivity of aqueous electrolytes, capacitors with high requirements for high frequency and low impedance in non-high temperature and high voltage environments often use high content of water in their electrolytes, but when a wider temperature range is required, they are used Non-aqueous electrolyte system.
6. Additives for working electrolyte
(1) Hydration of the electrode will increase the leakage current of the capacitor and reduce the capacitance. Adding a very small amount of phosphoric acid and its compounds can prevent the hydration of the oxide film, such as 0.05% to 5% alkyl phosphoric acid. Adding silicic acid compounds can also prevent electrode hydration. In addition, if the aluminum ions in the electrolyte reach saturation, the aluminum will be difficult to dissolve and hydration will not occur. Therefore, 0.01%~0.5% aluminate is sometimes added to the ammonium borate ethylene glycol series electrolyte to prevent Deterioration of electrolyte at high temperatures.
(2) Usually the oxide film on the surface of the aluminum foil will be damaged during the opening, riveting and winding processes of the anode aluminum foil, or under storage and working conditions. While the electrolyte repairs these dielectric films, hydrogen gas will be produced at the negative electrode, which will increase the internal pressure of the capacitor, shortening the service life of the capacitor, and even risking bursting at high temperatures. Therefore, thallium, indium, lead, bismuth and tin ions, which have greater adsorption force on the electrode, are generally added to the electrolyte to prevent corrosion and ionize hydrogen to prevent the internal pressure from rising. In addition, compounds containing phenol and nitro groups can also be added, such as resorcinol, nitrophenol, p-nitrobenzoic acid or p-nitrobenzyl alcohol and other hydrogen-absorbing substances. Due to the influence of the hydroxyl or nitro groups on the benzene rings of these substances, they are prone to hydrogenation reactions with hydrogen, thereby absorbing the generated hydrogen. The hydrogenation reaction is shown in Figure 1-1.
Figure 1-1 Hydrogenation reaction of p-nitrophenol (left) and p-nitrobenzoic acid (right)
(3) Chloride ions will seriously damage the electrode structure, and its reaction is as follows
![]()
and
![]()
Therefore, a small amount of nitro compounds, amide compounds, or a small amount of silver compounds or thallium compounds that can combine with chloride ions to form insoluble salts can be added to the working electrolyte to eliminate residual chloride ions in the electrolyte.
In order to prevent electrode corrosion, a small amount of boron oxide can generally be added to the working electrolyte.
(4) Improve low temperature characteristics. In order to reduce the low-temperature specific resistance and alleviate the deterioration of low-temperature performance, nitrous acid or nitrite, glycol ether, ethiol, ammonium p-nitrobenzoate, etc. are generally added to the electrolyte.
(5) Increase the flash voltage. In order to reduce the resistivity of the electrolyte and increase the flash voltage, ethylene oxide can be added to the ethylene glycol system to make it interact with water. In addition, esterification products of organic carboxylic acids and polyvalent alcohols or a small amount of compounds containing sulfinic acid groups can also be added.
(6) Improve the enabling characteristics. Adding a small amount of ammonium maleate, sorbitol and other empowerment accelerators can improve the empowerment properties.
Electrolyte of aluminum electrolytic capacitor—the above is an analysis of the composition of the working electrolyte
1.3 Electrolyte of aluminum electrolytic capacitor – basic characteristics of working electrolyte
1. Electrolyte of Aluminum Electrolytic Capacitor—Solvation effect in working electrolyte
The interaction between the solute and the solvent in the working electrolyte is called solvation, and the degree of solvation depends on the properties of the solute and the solvent themselves. Solvents are the basis of electrolytes. They can be divided into three categories based on their polarity, whether they can form hydrogen bonds, and the size of the empirical parameters of solvent polarity: ① Aprotic non-polar solvents, which have very weak interactions with solutes. Low viscosity. ② Aprotic polar solvents, molecular hydrogen is strongly bonded to other atoms and is difficult to give out protons, such as DMF, DMA. ③ Protic solvents, with acidic hydrogen, can easily form hydrogen bonds with entities that accept protons, such as water, ethylene glycol, etc. . Generally, protic solvents are good anionic solvators, and aprotic polar solvents are good cationic solvators. At a certain temperature, the strength of the solvation effect depends not only on the properties of the solvent (pH, dielectric constant, degree of polarization, etc.), but also on the charge size and shape of the solute ions themselves. The smaller the solute ion, the higher the charge density around it and the stronger the solvation effect. On the contrary, the solvation effect is weak. Below are solvation effects in two common electrolytes.
1)Ammonium adipate-ethylene glycol/water
The solvent is a mixture of ethylene glycol and water, which is a protic solvent and a good anionic solvator. The solute ammonium adipate has the following ionization equilibrium in the solvent:![]()
A hydrogen bond is formed between the proton of the solvent and the oxygen atom in the adipate ion, which solvates it, so the ionization equilibrium shifts to the right.
If the solute is ammonium formate, since the charge density of formate ions is higher than that of adipate ions, the solvation effect will be stronger in the solvent.
2) Triethylamine maleate-DMF
The solvent is DMF aprotic polar solvent, which is a good cationic solvator. The solute triethylamine maleate salt has the following ionization equilibrium in DMF:
![]()
Cation (:C2 H5) 3 NH+Easily solvated in DMF, shifting the ionization equilibrium to the right. When ethylene glycol is added to the DMF solvent, since it is an excellent solvent for negative ions, it can solvate maleate ions at the same time, making the overall solvation effect stronger.
2. Saturation vapor pressure of working electrolyte
The vapor pressure of the electrolyte directly affects the electrical performance and service life of the capacitor under high temperature conditions, so the electrolyte for high temperature operation needs to have a lower vapor pressure. Generally, the strength of the interaction between solute and solvent will affect the vapor pressure of the working electrolyte. The stronger the interaction force, the fewer molecules escape at the same temperature, and the lower the vapor pressure of the solution.
For electrolytes with ethylene glycol as the main solvent, generally in order to improve its conductivity, lower its freezing point, and improve low-temperature performance and viscosity, a small amount of deionized water needs to be added to ethylene glycol. However, after adding water, the vapor pressure increases significantly. When the water content is 10%, the solvent vapor pressure will rise from 0.933 kPa to 17kPa, as shown in Figure 1-2. Therefore, the proportion of deionized water needs to be adjusted according to the working temperature.
For electrolytes in which DMF is the solvent, the vapor pressure of DMF is as high as over 30kPa at 85°C. However, after adding the solute triethylamine maleate, triethylamine maleate ionizes into ions due to solvation in the solution. Keep the amounts of DMF and triethylamine constant. When the ratio of maleic acid to triethylamine is greater than 1:2, the maleic acid is excessive and the solution contains maleic acid molecules, which eliminates the ion-dipole interaction in the mixed solution. In addition to intermolecular interactions, there are also hydrogen bonds as shown in Figure 1-3. Due to the presence of hydrogen bonds, the interaction between the solute and the solvent is strengthened, so that the molecules in the mixed solution increase as the temperature increases, and the number of molecules escaping decreases, so the vapor pressure decreases.
When the ratio of maleic acid to triethylamine is less than 1:2, triethylamine is excessive and the solution contains triethylamine molecules. Because the polarity of triethylamine is relatively weak, the presence of triethylamine molecules further weakens the force of the solute solvent, thereby increasing the vapor pressure of the mixed solution. The temperature curve of vapor pressure is shown in Figure 1-4.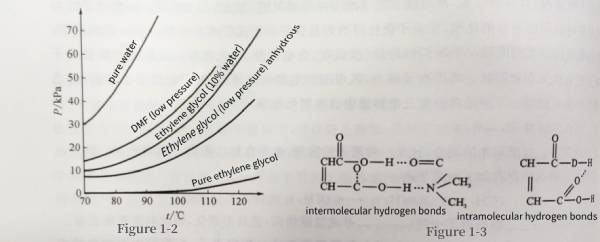 Figure 1-2 Vapor pressure of some solvents Figure 1-3 Hydrogen bonds produced when maleic acid is in excess
Figure 1-2 Vapor pressure of some solvents Figure 1-3 Hydrogen bonds produced when maleic acid is in excess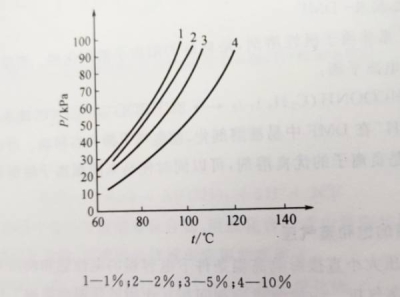 Figure 1-4 When DMF: triethylamine = 6:1, the vapor pressure-temperature curve of adding different contents of maleic acid
Figure 1-4 When DMF: triethylamine = 6:1, the vapor pressure-temperature curve of adding different contents of maleic acid
3. Impurity ions in the working electrolyte
The impurity ions in the working electrolyte are mainly chloride ions, sulfate ions and heavy metal ions, such as Cu2+, Fe3+
Chloride ions will seriously damage the electrode structure, leading to anode corrosion and a reduction in flash voltage. As shown in Figure 1-5, only 0.3×10-6chloride ions will have a significant impact on capacitor performance. Since chloride ions are widely present in sweat, dandruff, and dust, special attention must be paid to environmental hygiene when preparing electrolyte.
Sulfate ions will also corrode the anode and cause a decrease in flash voltage. However, since the corrosive effect of sulfate ions is not as strong as that of chloride ions, and it also has a certain oxidation effect, it can participate in repairing the oxide film, so the control requirements for sulfate ions are not as strict as those of chloride ions.
Heavy metal ions such as Cu2+and Fe3+ will form micro galvanic cells on the anode aluminum foil, corroding the aluminum foil, greatly increasing the leakage current of the product and reducing reliability. But it has little effect on flash fire voltage.
In order to remove impurity ions in the electrolyte, electrolysis or additive methods are generally used. The electrolysis method is to insert a graphite electrode into the electrolyte for direct current electrolysis, so that chlorine ions generate chlorine gas at the anode and escape, while heavy metal ions are deposited at the cathode; additivesThe rule is to add silver salts (silver acetate, silver benzoate, silver nitrate, etc.) to the electrolyte to precipitate gas ions.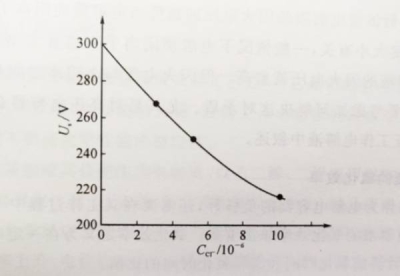
Figure 1-5 The relationship between the flash voltage of the electrolyte and the chloride ion concentration
Generally, the allowable impurity ion concentration in the electrolyte is:
Chloride ion<0.1x 10-6
Sulfate ion<0.5x 10-6
Heavy metal ions <1x 10-6
4. pH of working electrolyte
The pH of the working electrolyte has a great influence on the performance of the capacitor. This is because aluminum and aluminum oxides can be dissolved in both acid and alkali. When the pH value of the electrolyte is between 3.5 and 8.0, the Oxides are stable. On the other hand, the phosphate added to prevent water from damaging the media membrane can only stably generate aluminum phosphate and be effective when the pH value is >4.0. At the same time, the pH value will also affect its resistivity. Therefore, generally the pH value of electrolytes is placed in a range from neutral to slightly acidic.
5. Resistivity of working electrolyte
The size of the resistivity is related to the charge, number and movement speed of the ions in the electrolyte. The more ions in the solution, the lower its resistivity. When the number and charge of ions in the electrolyte are constant, the resistivity mainly depends on the speed of ion movement. When the viscosity of the solvent is high, the resistance to ion migration is great, and the ion movement speed slows down, so the conductivity of the electrolyte decreases. . The relationship between the conductivity and viscosity of the electrolyte conforms to Walden’s rule: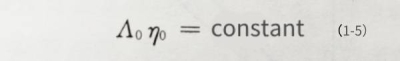
In the formula: Ao is the molar conductance of the electrolyte, and no is the viscosity of the pure solvent. Therefore, if you want to obtain a relatively stable resistivity, you must choose a solvent with a low viscosity.
6. Flash voltage of working electrolyte
The flash voltage of the working electrolyte has the following relationship with the resistivity of the working electrolyte:
![]()
In the formula: a, b are constants; p is the resistivity of the electrolyte; Us is the flash voltage.
Within a certain temperature, the flash voltage of a low-concentration electrolyte is related to the anion radius of the electrolyte, the solvation state and the viscosity of the solvent. Generally speaking, the larger the anion radius of the electrolyte, the better the solvation state, and the viscosity of the solvent. The higher the viscosity, the higher the flash voltage of the electrolyte. However, there is a contradiction between flash voltage and resistivity. Therefore, how to resolve this contradiction should be considered when selecting the working electrolyte. Especially for high-voltage capacitors, they must first meet the flash fire pressure. The details are described in the high-voltage working electrolyte.
7. Oxidation efficiency of working electrolyte
In addition to serving as the negative electrode of the electrolytic capacitor, the working electrolyte also needs to continuously repair the damaged dielectric oxide film during its working process. Therefore, the oxidation efficiency of the electrolyte is very important. Oxidation efficiency is defined as the ratio of the theoretically calculated oxidation time to the actual oxidation time when a certain current density reaches a certain enabling voltage: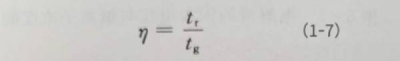
In the formula: tr is the time required for theoretical calculation; tg is the actual measurement time. The theoretical time required can be obtained from Faraday’s law of electrolysis:
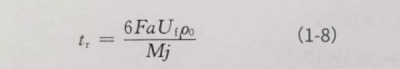
In the formula: F is Faraday’s constant; a is the energizing constant of A12 O3, which is about 1.2×10-7cm/V; Uf is the energizing voltage (V); p0 is the density of A12 O3, which is 3.42g/cm3; M is A12 O3The relative molecular mass of is taken as 102 g/mol; j is the current density mA/cm2and is substituted into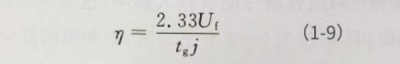
Figure 1-6 is the oxidation time curve of aluminum foil in two different electrolytes, with a current density of 2mA/cm2. According to the curve calculation, the oxidation efficiency of ammonium adipate-ethylene glycol electrolyte is about 49%, while the oxidation efficiency of maleic acid/triethylamine-DMF electrolyte is 62%, which is better than ammonium adipate -Ethylene glycol electrolyte.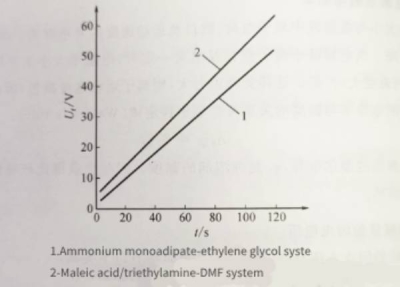
Figure 1-6 Oxidation time curve
Electrolyte of aluminum electrolytic capacitor—The above is an analysis of the basic characteristics of the working electrolyte
1.4 Electrolyte of aluminum electrolytic capacitor -Working electrolyte for low voltage
The preparation of the working electrolyte is one of the most critical processes for aluminum electrolytic capacitors. It is the actual cathode of the aluminum electrolytic capacitor and is the key to determining the life, reliability and electrical performance of the electrolytic capacitor. Therefore, the best combination of working electrolyte determines the working The stability of the physical and chemical properties of the electrolyte in various harsh environments.
At present, organic carboxylic acids or their salts are often used as electrolytes, and ethylene glycol, dimethylformamide, water, etc. are used as solvents. After adding various additives and mixing, changes in physical properties such as melting point, boiling point, viscosity, and polycondensation will inevitably occur. , acylation, cyanation and other chemical reactions. Especially due to the influence of electrochemical reaction and ambient temperature, the temperature of the working electrolyte increases, thereby accelerating the reaction. This results in increased viscosity, increased resistivity, deterioration of electrical properties, decreased capacitance, and even failure. Therefore, the optimal combination of working electrolytes is important.
1. General working electrolyte (-40~+85℃)
The low-voltage 6.3~63V electrolyte solvent is generally based on ethylene glycol, with an appropriate amount of water and dimethylformamide added. When ethylene glycol is used alone, its viscosity changes greatly with temperature, and alcohol polymerization and polyester reactions may occur, causing the electrolyte to deteriorate. Adding an appropriate amount of water can not only improve the physical properties of the electrolyte and provide oxygen for repairing the dielectric film, but also prevent the alcohol polymerization of ethylene glycol, polyester and other reactions to ensure the stable performance of the working electrolyte. In addition, it can also adjust the viscosity of the electrolyte. , reduce the cost of electrolyte. Water will produce hydration on the oxide film. If ammonium adipate is used as the solute, a trace amount of ADP needs to be added to prevent hydration. Phosphoric acid or hypophosphorous acid can also be used to inhibit hydration. Dimethylformamide has a high boiling point (153°C) and a low freezing point (-61°C). Adding dimethylformamide can increase the operating temperature range and improve temperature stability.
Commonly used solutes are adipic acid, formic acid or their ammonium salts. Adipic acid is a dibasic acid, and its ionization constant is much larger than that of boric acid. Therefore, a small amount of adipic acid can be used to obtain higher conductivity of the solute. Formic acid is the simplest acid among carboxylic acids. Its molecular weight is smaller than that of adipic acid, its ionization constant is large, and its molecular volume is small. Therefore, adding formic acid can effectively improve the conductivity of the working electrolyte. Ammonium adipate has good solubility in ethylene glycol and water, and has good empowering ability. Due to the large ionic radius of adipate, it can inhibit the hydration of the oxide film, increase the boiling point of the electrolyte, and reduce the vapor pressure. Ammonium formate has a small ionic radius and high ionization degree, so it has high conductivity and low viscosity. Especially at low temperatures, it can prevent the increase in viscosity of ethylene glycol and water from affecting the resistivity, but the amount added should not be too much.
2. Wide temperature working electrolyte (-55~+105 ℃)
With the development of electronic technology, higher requirements have been put forward for the operating temperature range of aluminum electrolytic capacitors, which can reach -55~+105°C. Therefore, wide-temperature operating electrolytes need to be studied. In addition to meeting the requirements of general working electrolytes, wide-temperature working electrolytes must also meet requirements such as high boiling point, low freezing point, low viscosity, flat resistivity temperature characteristics, and stable physical and chemical properties at high temperatures.
Maleic acid and its ammonium salt are usually used as solutes in wide-temperature working electrolytes. Maleic acid has relatively stable physical and chemical properties at high temperatures due to its special structure. Generally, an appropriate amount of amines (such as triethylamine) are added to neutralize it. Or partially neutralized to generate ammonium salt, which can not only adjust the pH value of the electrolyte, but also adjust the resistivity of the electrolyte. The maleate ions ionized in the solvent play a decisive role in repairing the oxide film. In addition to maleic acid, phthalic acid and benzoic acid are generally used as wide-temperature solutes.
The solvent of a wide-temperature operating electrolyte must remain liquid within a wide temperature range, so in most cases a mixed solvent of dipolar aprotic solvents and protic solvents that are miscible in different proportions is used. Commonly used solvents are mixed solvents of dimethylformamide (DMF) and ethylene glycol. In addition, N-ethylformamide (NEF) can also be used or added to N-methylformamide (NMF), dimethylformamide (DMF), N,N-diethylformamide (DEF) , the weight of NEF is not less than 10%.
Electrolyte of aluminum electrolytic capacitor—The above is the analysis of working electrolyte for low voltage
1.5 Electrolyte of aluminum electrolytic capacitor -Working electrolyte for medium and high voltage
1. Electrolyte of Aluminum Electrolytic Capacitor—General high-voltage working electrolyte
The medium voltage 100~160~250V working electrolyte mainly consists of adipic acid and its ammonium salts, benzoic acid and its ammonium salts as solutes, mannitol, ethylene glycol and a small amount of water as solvents.
The solute of the high-voltage 300-350~450V working electrolyte is mainly boric acid and ammonium pentaborate, linear or branched organic acids with more than eight carbons (such as suberic acid, sebacic acid, azelaic acid, etc.) and their ammonium salts , or 642 intermediate (Toyama’s intermediate or Zhoubang’s 1.6-DDA intermediate) as the solute, and add some organic acids and their salts as the second solute to increase the conductivity of the electrolyte. The solvent is usually ethylene glycol or mannitol.
The solute of the ultra-high voltage 500~600V working electrolyte is mainly boric acid and ammonium pentaborate, or 642 intermediate, and the solvent is mannitol or ethylene glycol and a small amount of diethylene glycol.
In addition to the solute and solvent, a small amount of phosphate can be added to the solvent to inhibit hydration, nitrophenol to absorb and produce hydrogen, or citric acid and ammonium dichromate to increase the flash voltage.
Citric acid has a macromolecular structure with three carboxyl groups and one hydroxyl group. Its large molecule is easy to polarize, so A12O3 is also very attractive to it. After adding citric acid, the citric acid is tightly adsorbed on A12O3to form an adsorption layer, which has the effect of shielding the electric field, thereby increasing the flash voltage.
After ammonium dichromate is added to the electrolyte, it will be ionized into Cr2 O72- under the action of the electric field. The negative ions Cr2 O72-will migrate to the anode. When the oxide film is damaged, Cr2 O72-will release atomic oxygen at the anode to repair the oxide film:![]()
This plays a role in increasing the flash voltage.
In addition, for ultra-high voltage working electrolytes, manufacturers sometimes add additional voltage boosting agents and anti-corrosion agents.
2. Medium and high voltage wide temperature working electrolyte
For medium and high-voltage wide-temperature working (-40~+105℃) electrolytes, in addition to meeting the requirements of low-voltage wide-temperature working electrolytes, they must also have a sufficiently high flash voltage. Therefore, the solute generally chooses succinic acid, adipic acid or sebacic acid and its triethylamine salt, which dissociates into acid anions in the solvent and has strong empowering ability and high stability. The solvent is mainly ethylene glycol, mixed with DMF and y-butyrolactone to improve the high and low temperature characteristics of the electrolyte.
The working electrolyte used at higher temperatures (125°C) uses tert-butylamine adipate salt and dipropylamine adipate salt as the solute, ethylene glycol and water are mixed as the solvent, and phosphate is added to prevent hydration. Alternatively, ammonium dodecanedioic acid salt can be used as the solute, and a mixture of water, ethylene glycol and N-methyl-2-pyrrolidone (NMP) can be used as the solvent. The boiling point of NMP is 202°C, the flash point is 95°C, and the autoignition point is 346°C. Adding NMP to ethylene glycol will increase the solubility of the salt and increase the conductivity. The electrolyte based on this solvent has a resistivity of 707Ω·cm at 25°C and a maximum voltage of 462V.
Electrolyte of aluminum electrolytic capacitor—The above is the analysis of working electrolyte for medium and high voltage
1.6 Working electrolyte of aluminum electrolytic capacitor for motor starting
Aluminum electrolytic capacitors used for starting electric motors usually work under AC high voltage and large load. The capacitor itself generates significant heat. If the tangent value of the loss angle is too large, the electrical properties of the capacitor will easily deteriorate or even fail due to the large amount of heat generated. At the same time, at the moment of startup, the capacitor is subjected to instantaneous high voltage. If it is higher than the flash voltage, it may cause dielectric breakdown. Therefore, when selecting the working electrolyte for this kind of capacitor, the primary criteria are good high temperature stability, small loss tangent value tan δ and high flash voltage.
First of all, the electrolyte needs to have a high boiling point and low vapor pressure to reduce the volatilization of the electrolyte and prevent explosion during high-temperature operation. Boiling points can be significantly increased using waterless and water-less systems. Secondly, the loss tangent value mainly depends on the permeability coefficient of the paper. Generally, the higher the viscosity of the electrolyte, the lower the permeability coefficient. Therefore, reducing the viscosity of the electrolyte can enhance its infiltration into paper, thereby reducing the loss tangent. Finally, using macromolecular solvents, the anion radius after solute ionization is large, and a thick enough anion solvent layer is formed on the anode surface to shield the electric field, so that the flash voltage can be increased.
Based on the above requirements, the triethylamine adipate-ethylene glycol system is generally used for 110V capacitors, and the ammonium pentaborate-ethylene glycol system is used for 220V capacitors.
Electrolyte of aluminum electrolytic capacitor—The above is the analysis of the working electrolyte of aluminum electrolytic capacitors used for motor starting.
1.7 Electrolyte of aluminum electrolytic capacitor -Working electrolyte with special requirements
1. Low resistivity working electrolyte
In recent years, the increase in the specific volume of aluminum foil has created the most favorable conditions for the miniaturization of aluminum electrolytic capacitors. As the specific capacitance increases, if the traditional working electrolyte continues to be used, the loss tangent value of the product will exceed the standard. , so if you want to develop small-volume and ultra-miniature products, you must significantly reduce the resistivity of the working electrolyte.
In terms of low voltage, the resistivity of an electrolyte using DMF as the main solvent and 10% mass ratio of 2,6-dimethylpyridine as the solute can be as low as 45.5Ωcm. A 25 V/10μF electrolytic capacitor made with this electrolyte After 1,000 hours of high-strength load testing at 105°C, the electrical properties remain excellent. In addition, the electrolyte using N-methylformamide as the solvent and a mixture of 15% nicotinic acid and N,N-dimethylpyridinediamine as the solute has a resistivity as low as 54Ω·cm at 30°C. The manufactured 16 V/180muF electrolytic capacitor has been subjected to a high temperature load test of 105°C for 1000 hours, and its electrical properties remain excellent.
In terms of medium and high voltage, the electrolyte using 75.3% DMF and 4% pure water as solvents, 5.4% glacial acetic acid, 7% boric acid and 8.3% diethylamine as solutes has a resistivity of 217Ω·cm at 25°C. The fire voltage can reach 500V, and it has strong ability to repair the oxide film of aluminum foil. In addition, using 80% ethylene glycol as the solvent, 10% 1-methyl-1,7-dimethyldiammonium pimelate as the solute, and adding 10% polyglycerol to the electrolyte, the resistivity at 30°C It is 460Ω·cm, and the flash voltage is as high as 510V. The 400V/270μF product made with this electrolyte has maintained excellent electrical properties after a 1000-hour high-temperature load test at 105°C.
In addition, you can choose organic carboxylic acids with large three-dimensional structures, such as pimelic acid, suberic acid, azelaic acid, sebacic acid or dodecanedioic acid. Combined with triethylamine, tributylamine, isopropylamine or aniline as solute. The longer the carbon chain of organic carboxylic acids and organic amines, or the more branched chains or benzene rings they have, the higher the flash voltage of the prepared electrolyte.
2. Working electrolyte for cleaning-resistant aluminum electrolytic capacitors
Cleaning-resistant aluminum electrolytic capacitors refer to capacitors that can withstand cleaning with organic halogenated hydrocarbon solvents. In order to remove residual flux from printed circuit machines, foreign complete machine manufacturers widely use halogenated cleaning agents such as Freon and chloroform. Halogenated hydrocarbon organic cleaning agents are highly active and have a high degree of penetrating ability. They can penetrate capacitor sealing materials, causing corrosion of the capacitor aluminum foil and causing capacitor failure.
In order to make the electrolytic capacitor more resistant to cleaning by halogenated hydrocarbon solvents, in addition to using sealing materials with strong resistance to solvent penetration, it is also necessary to add a small amount of special chemical reagents to the electrolyte. This reagent is referred to as a cleaning-resistant additive. Commonly used cleaning-resistant additives are nitro compounds (such as m-nitrophenol, m-nitrobenzene, nitrobenzoic acid), ketones (methyl phenyl ketone, diphenyl ketone, etc.) and carboxylic acid complexes ( Such as nitrotriacetic acid, ethylenediaminetetraacetic acid, etc.). The added cleaning-resistant additive can form a complex with halide ions, thereby preventing halide ions from escaping and corroding the oxide film on the aluminum foil. Aminotriacetic acid also has the ability to resist the hazards of chloride ions.
The content of cleaning-resistant additives cannot be too high, otherwise it will cause the tan δ value of the capacitor to increase and the flash fire voltage to decrease. Generally speaking, the mass percentage of cleaning-resistant additives in the electrolyte should not exceed 1%.
3. Working electrolyte for long-life electrolytic capacitors
Long-life electrolytic capacitors refer to products that can withstand at least 2000h of durability testing at the specified upper limit category temperature.
Since DMF has a low viscosity and is easily volatile, the electrolyte using DMF as a solvent has a short service life. One of the ways to improve its life characteristics is to add γ-butyrolactone. When the mass ratio of γ-butyrolactone to DMF exceeds 1/3, its characteristics can be improved. When the content of γ-butyrolactone is high, it is beneficial to extending the life span. This is beneficial to the product’s lifespan and to further improving its low-temperature characteristics. However, the price of y-butyrolactone is relatively high, and its price is 30 to 40 times that of DMF. Another economical and effective way to extend life is to mix ethylene glycol into the solvent, which mainly takes advantage of the high viscosity of ethylene glycol.
On the other hand, the electrolyte with organic ammonium carboxylate as the solute may undergo the following amidation reaction at high temperatures:
![]()
The electrolyte using organic ammonium carboxydioate may also undergo amidation reaction at room temperature. To ensure the long life of the capacitor, the amidation reaction of the electrolyte must be prevented. Because the amidation reaction generates water as a by-product, the aluminum foil is corroded and AI(OH)3 is produced, which increases the leakage current of the product and even causes product failure.
There are two main methods to inhibit amidation of the working electrolyte: the first method is to add chemicals containing acyl groups, such as adipamide; the other method is to use three-dimensional structures such as alkylcarboxylic acid or phenylcarboxylic acid. When large organic acids react with organic amines, the salts generated will not cause amidation reactions due to dehydration.
Electrolyte of aluminum electrolytic capacitor—The above is the analysis of working electrolyte for special requirements.
Summarize
Electrolyte of aluminum electrolytic capacitor—This article mainly talks about the property requirements of working electrolyte, the composition and basic characteristics of electrolyte, and the analysis of working electrolyte for low, medium and high voltage.To learn more about capacitors, please click:https://solidcapacitor.com




